Immune Cell Activation was selected by ESA in 2019 following the open Call for commercially-driven research projects.
At the core of the idea lies the ambition to use magnetic nanoparticles for therapy and diagnosis of diseases such as cancer, and to overcome current limitations of Cancer stromal targeting (CAST) therapy.
Using the space environment, and in particular conditions of microgravity, the project team plans to study the uptake of the nanoparticles by t-cells which aims to improve delivery mechanisms on Earth.
The Immune Cell Activation project is part of the Biomedicol Initiative coordinated by Colorobbia Consulting S.r.l. in collaboration with the University of Florence and Kayser Italia who offer access to space through the commercial Bioreactor Express Service.
OBJECTIVES
The objective of the project is the development of a construct based on T lymphocytes, customized for an individual patient, strengthened in its primary functions by the presence of a magnetic core inside and able to generate hyperthermia in situ.
In order to increase the knowledge related to the stability of the nanoparticles, their compatibility with the immune system and their ability in loading the lymphocytes and the knowledge of the behavior of the “armed” lymphocytes themselves, it is planned to observe the main constituents of the “construct” , the cells and the nanoparticles in conditions of microgravity.
The Patient at the Centre of Personalised Medicine
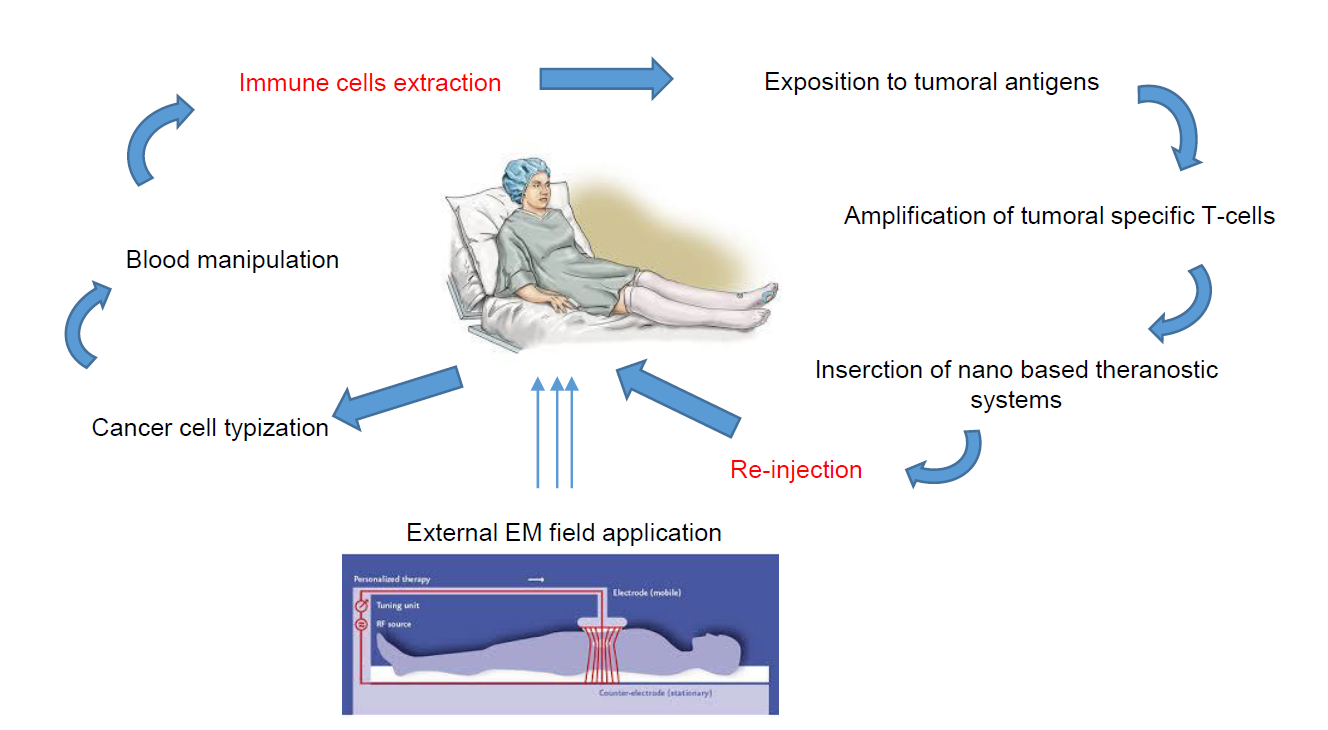
Exposure to microgravity will help the evaluation of the final protocols of a melanoma cancer treatment in an in vivo animal test that will be conducted on the ground after the conclusion of the project. The acquired data related to the different checkpoints involved in the activation of immune cells (T and B cells) by the nanoparticles and their internalization within the immune and melanoma cells will also be released.
During the microgravity research phase, the goal is to:
- evaluate the ability of T or B cells to load nanoparticles,
- unravel the cellular and molecular aspects of T-B cell interactions and activation in the presence or absence of nanoparticles in the space environment, and to
- evaluate the ability of A375 melanoma cells to internalize nanoparticles.
This is expected to solve the main problems related to:
- the strong tendency of nanoparticles to sediment, and
- the increase of nanoparticle loading in cells by studying long-term incubation in microgravity.


SCIENCE
The immune system
The body has many types of immune cells to help combat disease. One such type is called T-lymphocytes, or “T-cells”. T-cells have receptors that are able to recognize specific proteins, called “antigens”, on the surface infected or cancerous cells. This binding between receptor and antigen induces a chemical chain reaction inside the cell that causes the T-cell to kill the infected or cancerous cell. Each T-cell can only develop receptors able to recognize one specific type of antigen. If a T-cell has developed its specific receptors, it can be referred to as a “specific T-cell”.
Another type of immune cell you’ll see mentioned here are B-cells, the cells responsible for producing antibodies. Finally, you will also see mentioned “melanomas”, which are the most serious type of skin cancer cells.
Engineering a T-cell to become a more efficient killer:
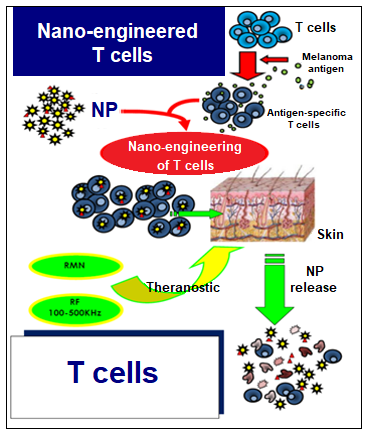
The study aims to develop a “construct” made of T-cells and magnetic nanoparticles.
The T-cells used in the experiment are “specific” T-cells taken from the patient diagnosed with melanoma. This means that these T-cells contain the receptors that are specific to the antigens on the surface of the patient’s melanoma cells.
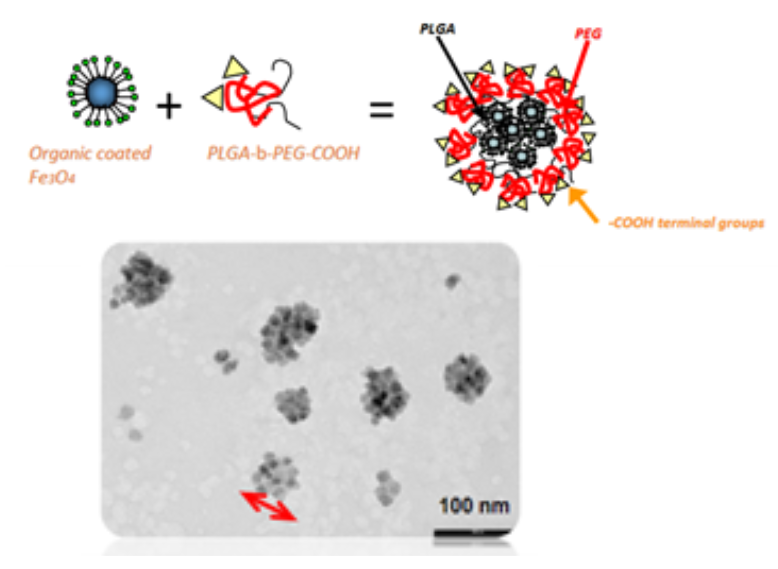
The nanoparticles are up-taken into the T-cell’s cytoplasm (a gel-like fluid inside cells where most chemical reactions happen) where they stay in a stable state. Once the nanoparticles have been up-taken by the T-cell and the construct is complete, the T-cell is now said to have been “nano-engineered”.
The reason behind this nano-engineering is simple. The “activation” of the T cell killing pathway can be induced by heat. Once the T-cells have the magnetic nanoparticles inside, an electromagnetic field is beamed onto the cells, which causes the nanoparticles to heat up within the T-cell’s body. This generates a “local” heat (or local hyperthermia) that only activates the killing pathway of that specific nano-engineered T-cell.
BENEFITS
What are the benefits of this research?
If we now consider this T-cell/nanoparticle construct to be inside the human body, where the nano-engineered specific T-cells have been re-introduced into the patient’s blood stream, it is easy to imagine how this treatment can be so advantageous over other forms of cancer treatments.
Unlike chemotherapy or other radiation treatments, which are harmful not only to cancerous cells but also to other cells in our system, the electromagnetic field that is used is harmless to the human body. Moreover, the use of specific T-cells makes the treatment highly accurate, targeting only the melanoma cells, and the fact that these T-cells originate from the patient makes the treatment highly personalized without the risk of rejection by the body.
Finally, after the T-cells are activated by the application of the electromagnetic field, the nanoparticles are released in a harmless way and get recycled by other cells from the immune system, making this a zero side-effect treatment.
Better quality of life for patients and their families
The expected administration of this treatment is 3-4 times over the course of 3-5 years, making it a minimally invasive care treatment, reducing the patient’s time spent at care facilities.
The high efficacy of the treatment combined with zero side effects also greatly impacts the life quality of the patient as well as of the family and loved ones around them.
It’s worth remembering that this type of treatment can also be used for patients with CNS (central nervous system) diseases such as multiple sclerosis, Alzheimer and Parkinson’s disease.
THE ADDED VALUE OF SPACE
All levels of biological organisation, including cells, tissues, organs, and organisms, are affected by space conditions, especially that of microgravity, often in novel and useful ways, sometimes in ways that allow medical, biotech and other problems on Earth to be better addressed. The benefits of space conditions allow us to manufacture medicines, materials and living tissue that cannot be done in Earth’s 1g environment.
Space offers us the possibility to explore all the conditions to improve the science behind the experiment. This project will allow us to explore pathways we couldn’t explore in a normal lab – Constanza Ravagli, PhD, Senior Scientist at Colorobbia
Three reasons why the ICA experiment is being conducted in space:
1. One of the main issues encountered when running this experiment on the ground was the tendency for the nanoparticles to sediment in their biological solution, which reduces their uptake by T-cells. The researchers are expecting that in space this problem will be solved, as there is no sedimentation in microgravity, thus increasing the efficiency of the nanoparticles’ uptake.
2. Microgravity may as well act on the chemistry aspect of the nanoparticles, in a way that can also help them enter the T-cells more efficiently.
3. It is well known that the space environment can alter the expression of certain genes. Many genes related to nanoparticle uptake are up-regulated in space (meaning an increase in the expression of these genes). This makes the researchers believe that space conditions will be an ideal setting for the uptake of nanoparticles by T-cells, and it could also reveal some molecular pathways related to cellular uptake that otherwise are hidden under the effects of gravity
Once back on Earth, the samples will have their RNA (a precursor of DNA) analysed, and the identified microgravity-affected genes will then be targeted in future experiments, in order to try and replicate the improvement of the nanoparticle uptake in space.
TAKING MEDICAL RESEARCH TO SPACE
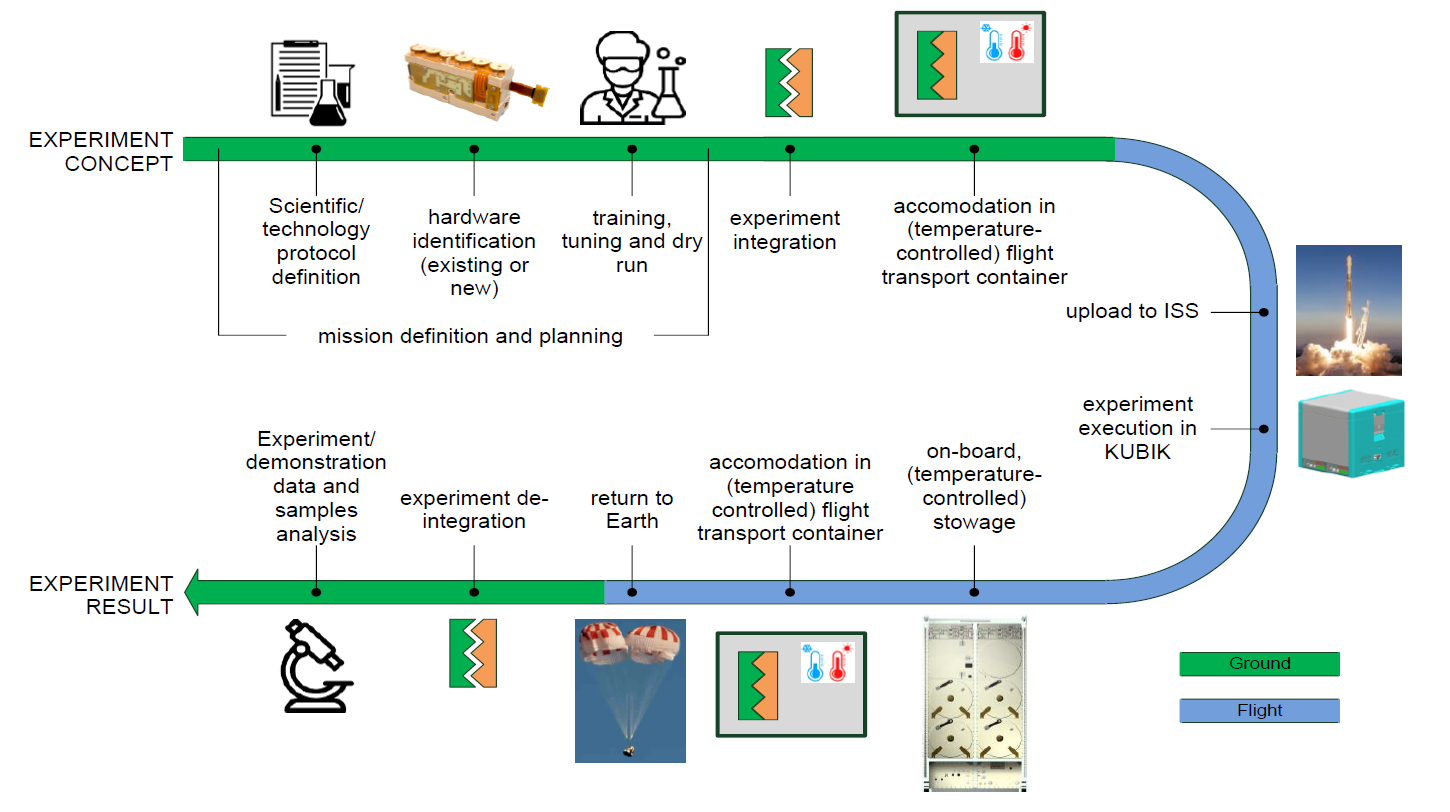
To send the project to space, the project is making use of the commercial Bioreactor Express Service which provides access to the ESA-owned KUBIK Facility aboard the International Space Station (ISS).
Steps to prepare the project for flight include:
- Preparation of the tailored nanoparticles
- Bioreactors Ground Models refurbishment and readiness for ground experiments
- Establishment of protocols for culturing of T cells, B cells and melanoma cells inside bioreactors and nanoparticle uptake
- Consolidation of scientific protocols and readiness for flight
- Mission preparation
- Flight campaign
- Post flight analysis
During the end-to-end project, the team received support from Bioreactor Express provider Kayser Italia which has more than 20 years experience in delivering biological and life science related projects to space.

NEXT STEPS
The steps following the IMM project, which is essential for the future market introduction of nanoparticles suitable for this purpose, will be as follows:
- Execution of in vivo POC, exploiting the IMM results.
- Execution of Toxicity and Safety tests
- Application for authorization for use on humans to EMA
- Execution of Clinical Phase I, II III
- Marketing Authorization (Phase IV) by EMA.
Phases 1-5 will be conducted by Colorobbia over a period of 5 to 10 years depending on the responses of the regulatory agencies.
[wp_ulike]


